With unpredictable risk and profound fatigue, Cold Agglutinin Disease (CAD) is a severe disease with high unmet need1-5
For patients with CAD, the effects of chronic hemolysis can cause acute consequences and chronic risks
CAD is a rare, complement-mediated, autoimmune hemolytic anemia with acute consequences and chronic risks.1-3 Hemolysis in CAD is driven by C1 activation of the classical complement pathway, when cold agglutinins (IgM autoantibodies) recruit and activate C1, typically at body temperatures under 98.6 °F (37 °C).6,7 Get a deeper look at the mechanism of hemolysis in CAD and how ENJAYMO works.
“CAD is an insidious disease. I looked perfectly normal, but it was silent, sapping my strength…my energy level just dropped.”
Unmet needs remain for patients despite management with cold avoidance and therapies not indicated for CAD
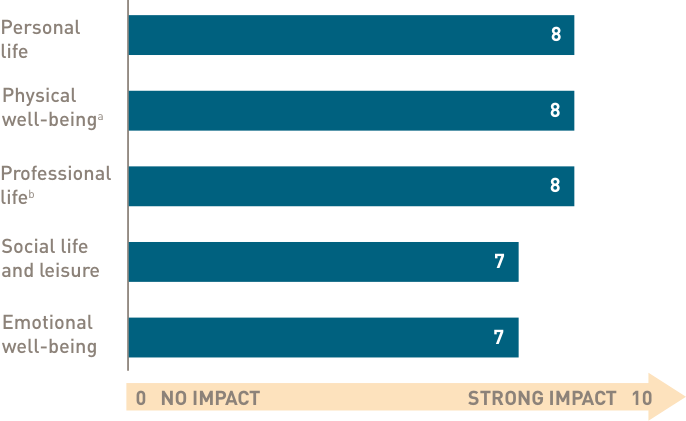
Patients with CAD endure an invisible burden of profound fatigue5
In a US patient survey, fatigue is the CAD-related symptom patients self-reported as having the greatest impact on their daily lives (45/50)‡
Impact of fatigue on daily life from a prospective survey of patients with CAD (N=50)
- *Therapies included corticosteroids, danazol, ibrutinib, immunoglobulin, rituximab, immunosuppressants, antineoplastics, plasma exchange, splenectomy, and other biologics (eg, eculizumab and lymphocyte immune globulin antithymocyte globulin).
- †Study patients with CAD were initiated on therapies including corticosteroids, IV immunoglobulin, rituximab, immunosuppressants, antineoplastics (eg, bendamustine), or biologics (eg, eculizumab).
- ‡Data from a self-administered internet-based survey are likely to be biased toward patients with access to the internet and a computer (ie, a bias to younger and socioeconomically advantaged patients). A recall bias and reporting from memory may result in an underestimation of certain clinical information.
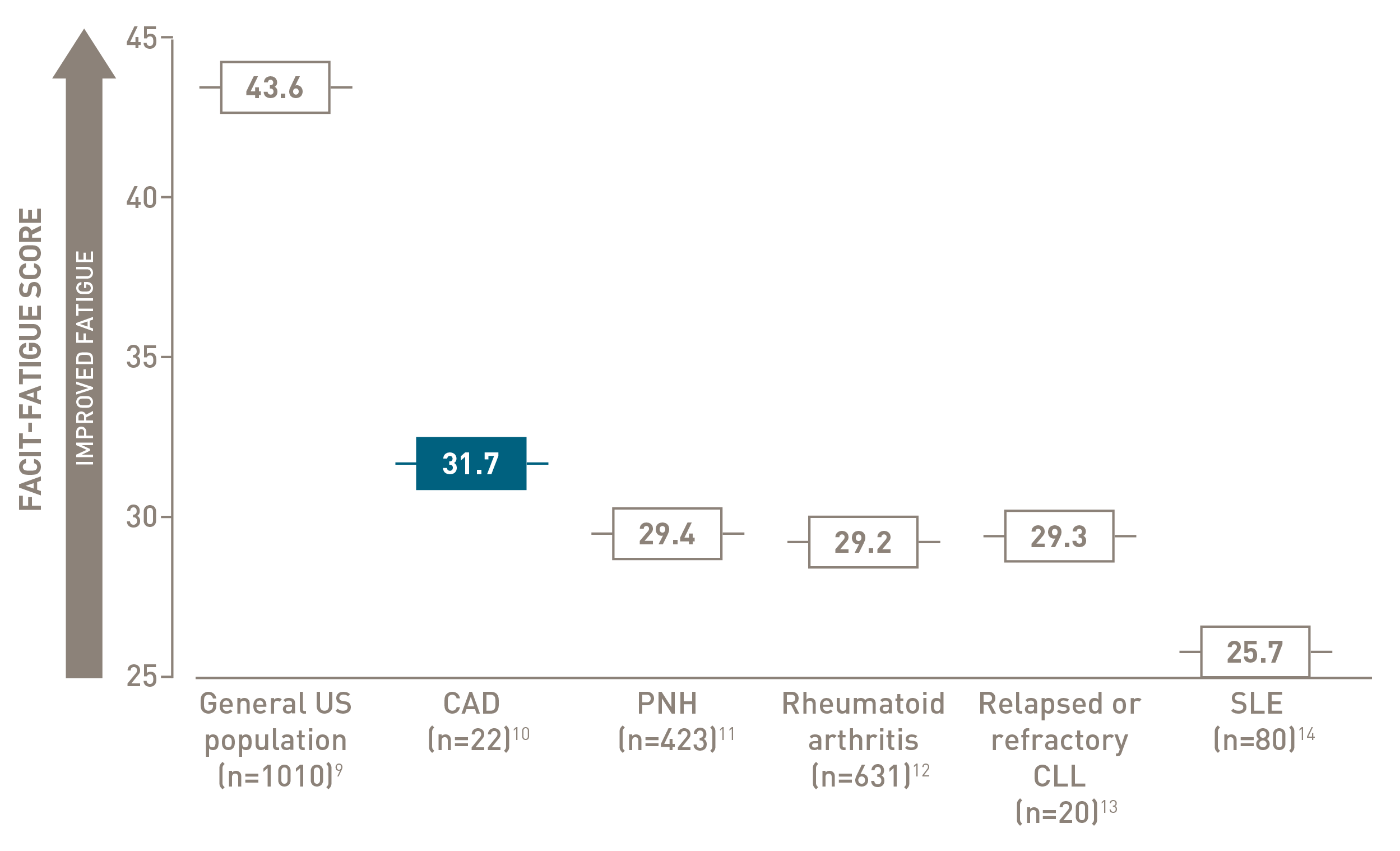
FACIT-Fatigue is a patient-reported outcome instrument with scores ranging from 0 to 52, with higher scores indicating less fatigue.
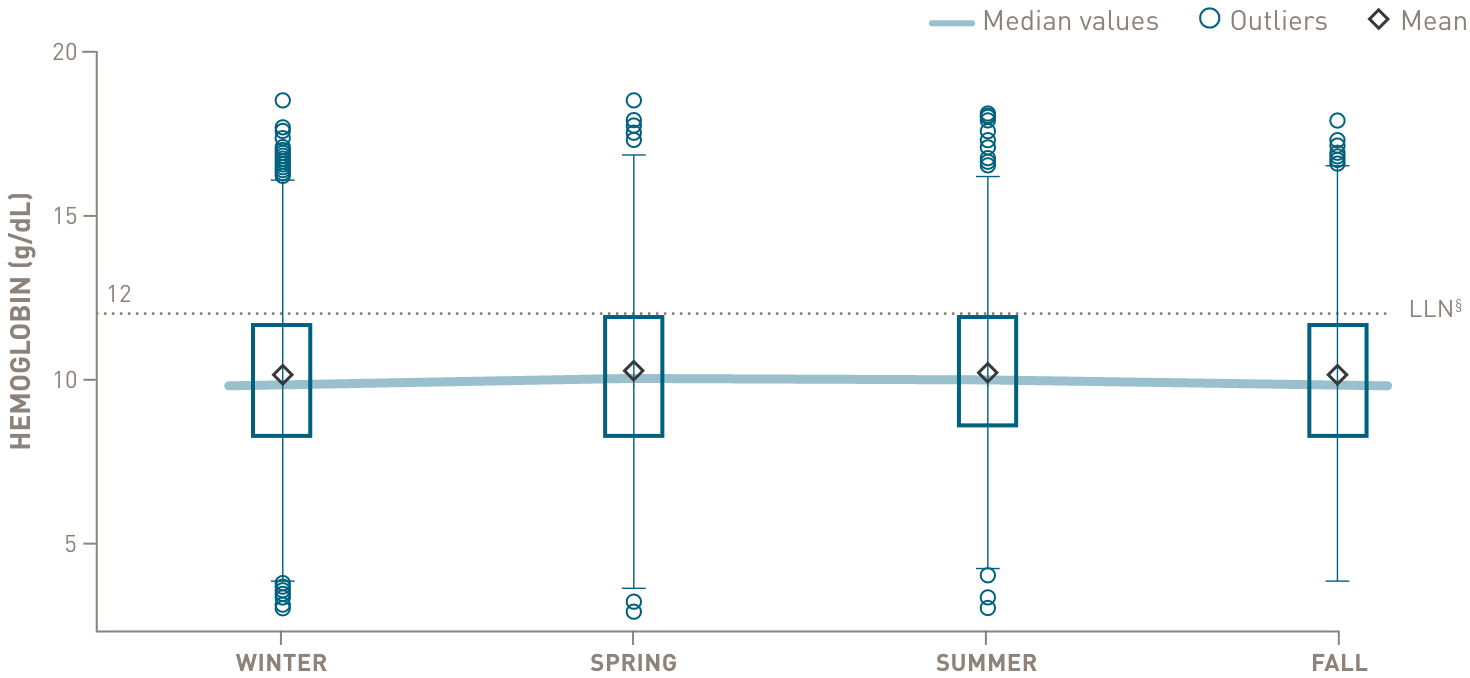
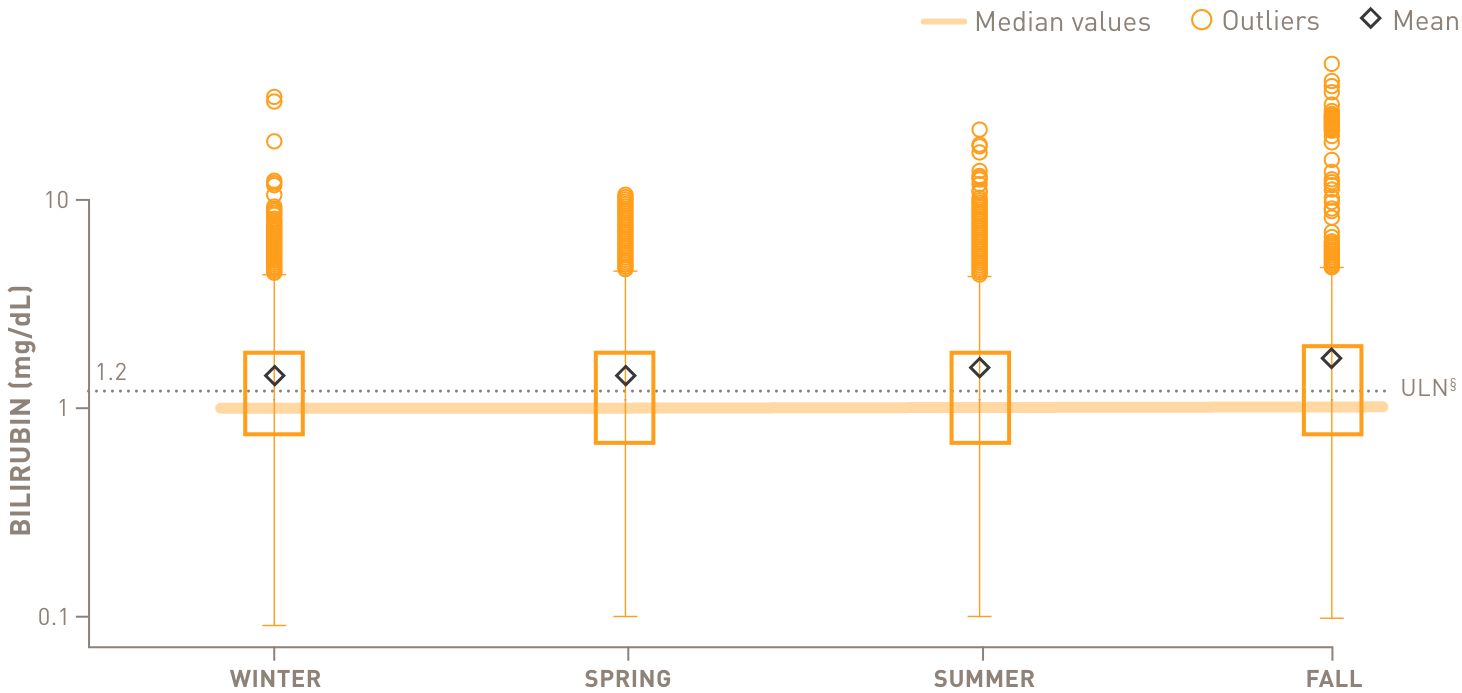
Chronic complement-mediated hemolysis in CAD drives year-round anemia, regardless of season15
In a retrospective analysis of patients with CAD (n=594)
Mean values of hemoglobin and bilirubin levels by season
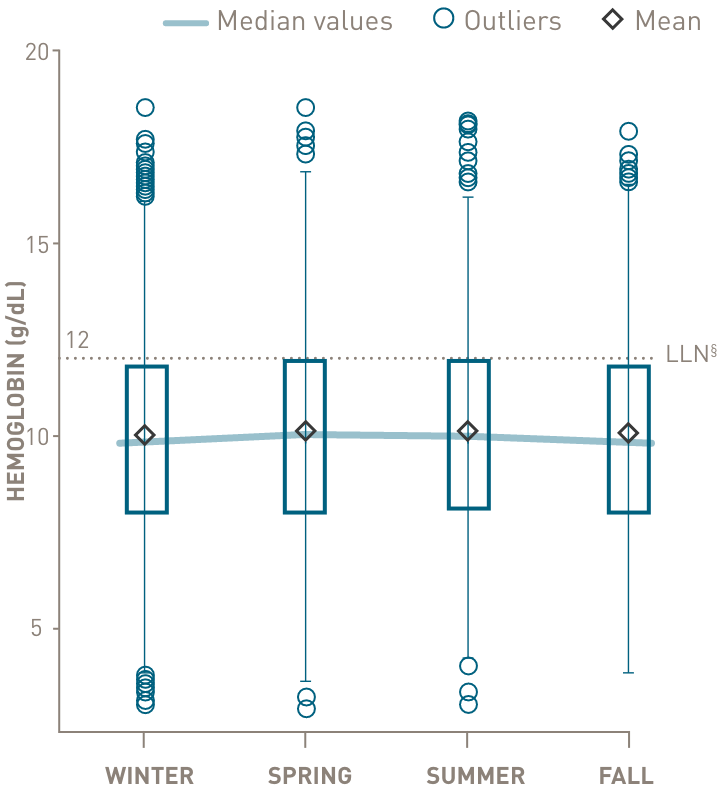
below the lower limit of normal
through all 4 seasons
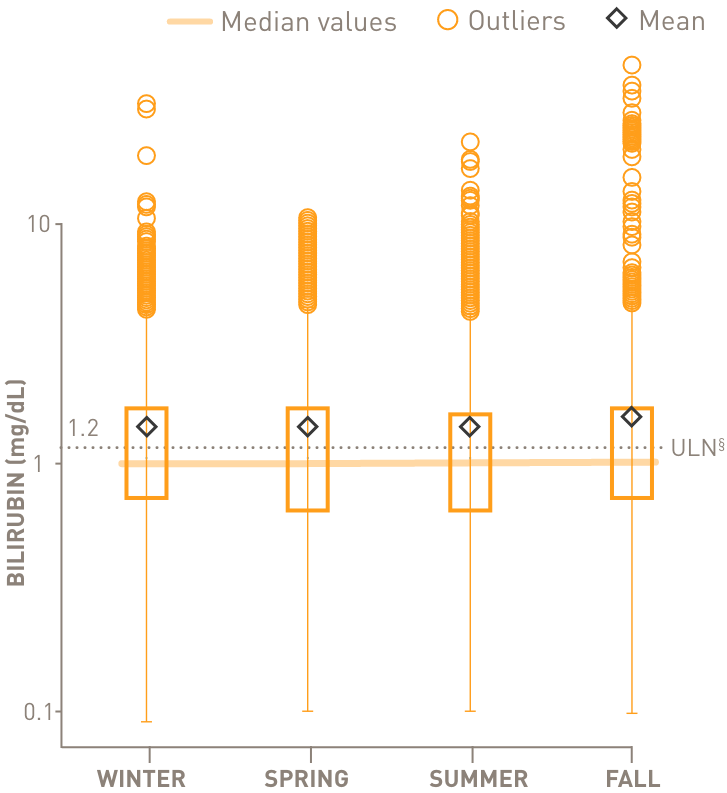
above the upper limit of normal
through all 4 seasons
The estimated median value for LDH was above normal for all 4 seasons
- §Normal hemoglobin levels for adults vary, but in general are 14 to 17 g/dL for males and 12 to 16 g/dL for females. Normal bilirubin is 0.3 to 1.2 mg/dL.