ENJAYMO is a breakthrough in the treatment of Cold Agglutinin Disease (CAD)1
ENJAYMO is indicated for the treatment of hemolysis in adults with CAD.
CADENZA, the first placebo-controlled trial in CAD, demonstrated a significant benefit for patients treated with ENJAYMO1,2
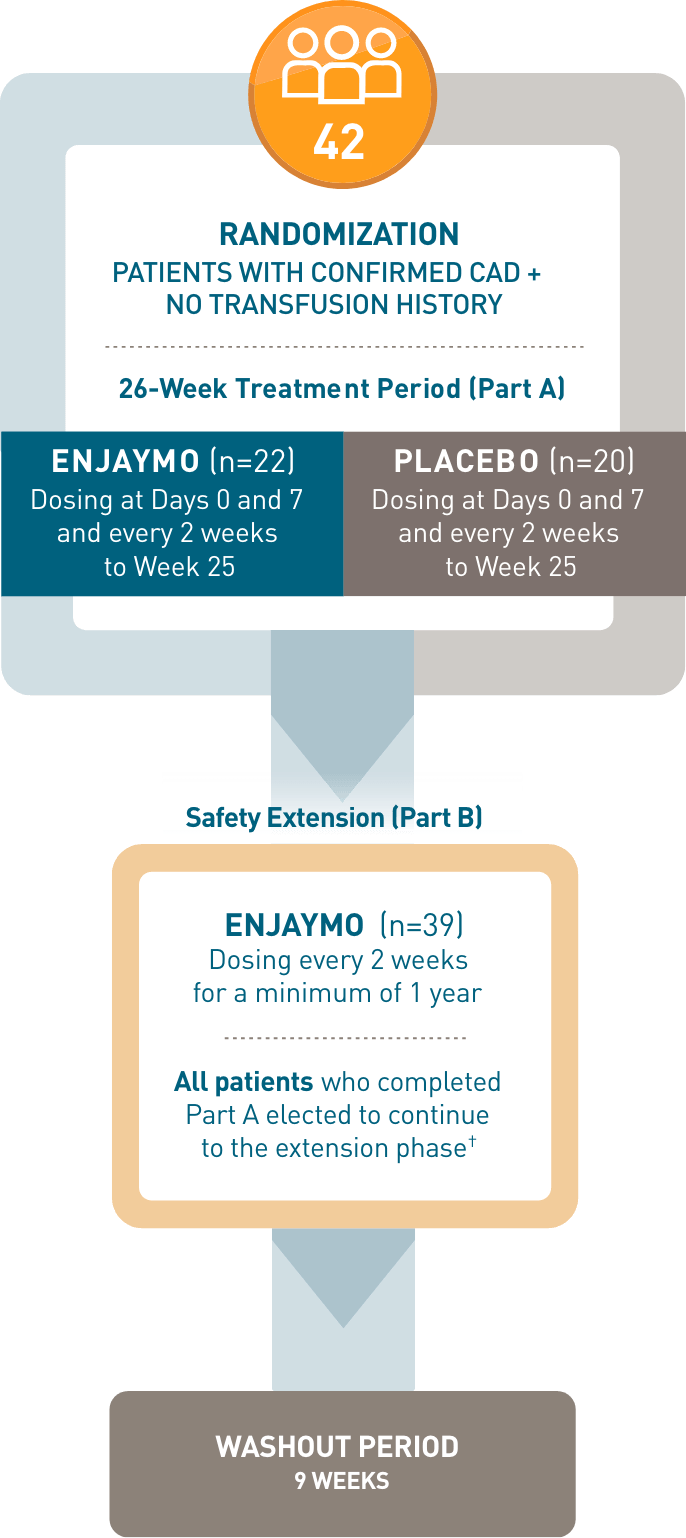
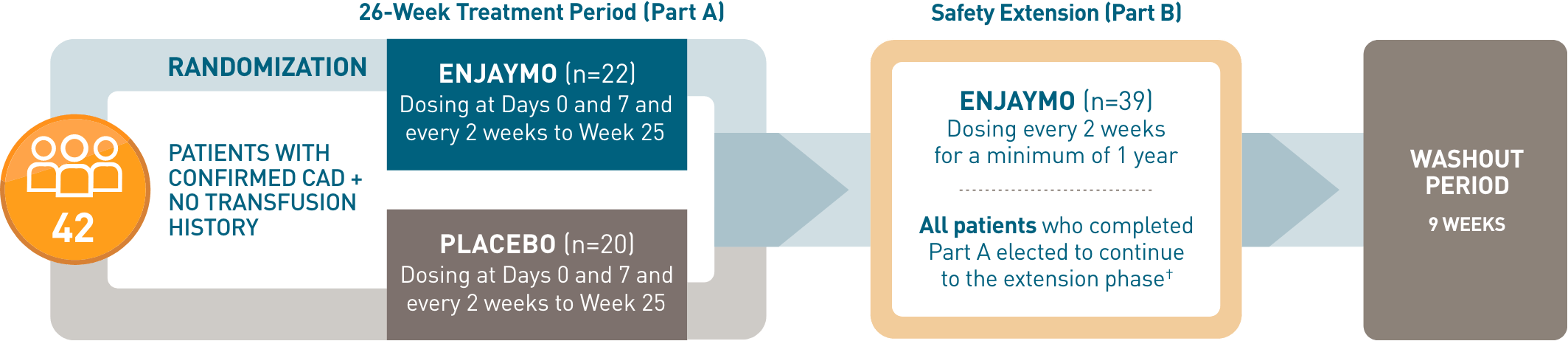
CADENZA, a phase 3, placebo-controlled, global, multicenter, randomized, double-blind trial, demonstrated efficacy and safety of ENJAYMO in 42 patients with CAD and no transfusion history.1-3*
CADENZA, a phase 3, placebo-controlled, global, multicenter, randomized, double-blind trial, demonstrated efficacy and safety of ENJAYMO in 42 patients with CAD and no transfusion history*
ENDPOINTS STUDIED
COMPOSITE PRIMARY ENDPOINT
No use of protocol-prohibited CAD treatment from Weeks 5 to 26
KEY SECONDARY ENDPOINTS
-
Effect on Hb (mean change from baseline at TAT)
-
Change in fatigue (mean change from baseline at TAT in FACIT-Fatigue score)
Laboratory measures of hemolysis (mean change from baseline at TAT in bilirubin and LDH)
- *Patients with confirmed CAD and no history of transfusion within 6 months or >1 in 12 months prior to enrollment (N=42). Patients with CAS secondary to infection, rheumatologic disease, SLE, or overt hematologic malignancy were excluded, whereas patients with a history of or concomitant low-grade lymphoproliferative disease (bone marrow involvement <10%) were not excluded.
- †In CADENZA Part A, 3 patients discontinued treatment in the ENJAYMO arm and did not complete the trial. All 39 patients who completed Part A continued to Part B.
- ‡Treatment assessment time point (TAT) was defined as the mean value from Weeks 23, 25, and 26.
- FACIT-Fatigue is a patient-reported outcome instrument with scores ranging from 0 to 52, with higher scores indicating less fatigue.
| Parameter | Statistic |
Placebo n=20
|
ENJAYMO n=22
|
|---|---|---|---|
| Age | Mean (min, max) |
68.2 (51, 83) |
65.3 (46, 88) |
| Sex Male Female |
n (%) | 4 (20.0) 16 (80.0) |
5 (22.7) 17 (77.3) |
| Body weight | Mean, kg (min, max) |
64.9 (48, 95) |
66.8 (39, 100) |
| Hemoglobin | Mean, g/dL | 9.33 | 9.15 |
| Bilirubin (total)§ | Mean, mg/dL | 2.09 (1.75 × ULN) |
2.41 (2 × ULN) |
| LDH | U/L | 380.8 | 421.5 |
| History of transfusion Within last 6 months Within last 12 months |
Median (range) | 0 0 |
0 0.14 (0, 1) |
| FACIT-Fatigue scale‖ | Mean | 32.99 | 31.67 |
- §Bilirubin data excluding patients with either a positive or no available test for Gilbert’s syndrome (n=18 for placebo, n=20 for ENJAYMO).
- ‖Fatigue is measured on a scale of 0 (worst fatigue) to 52 (no fatigue).
ENJAYMO is the first and only treatment for CAD, with a proven and significant benefit vs placebo across key efficacy measures1,2
The majority of patients achieved an improvement in hemoglobin and transfusion independence with ENJAYMO and required no additional CAD treatment (16/22)
achieved all 3
composite endpoint measures¶
- Significant hemoglobin increase
- Transfusion independence
- No additional treatment used#
vs 15% (3/20) with placebo
(Responder rate difference: 58.8%,
95% CI: 34.6%-83.0%, P=0.0004)
Treatment assessment time point (TAT) was defined as the mean value from Weeks 23, 25, and 26.
¶Two patients discontinued prior to Week 23, and their status was considered unknown for the purposes of this analysis.
#Prohibited therapies included rituximab alone or in combination with cytotoxic agents.
Controlling complement-mediated hemolysis in CAD starts with the first dose of ENJAYMO1,2
Mean Hb and bilirubin levels through Week 26 with ENJAYMO vs placebo (N=42)**
**Mean baseline values: Hb was 9.15 g/dL for ENJAYMO and 9.33 g/dL for placebo; bilirubin was 2.41 mg/dL for ENJAYMO and 2.09 mg/dL for placebo. Improvement at TAT: LS mean change in Hb was 2.66 g/dL for ENJAYMO and 0.09 g/dL for placebo; mean change in bilirubin was –1.29 mg/dL for ENJAYMO and –0.11 mg/dL for placebo.
Patients experienced significant improvement in the symptoms and impact of fatigue at TAT1,2
Mean FACIT-Fatigue scores through Week 26 with ENJAYMO vs placebo (N=42)††
Data at TAT (mean values from Weeks 23, 25, and 26) were tested for significance. Between baseline and Week 26, data at each time point were the observed mean. Interpret these data with discretion given small sample.
††Mean baseline score for FACIT-Fatigue was 31.67 for ENJAYMO and 32.99 for placebo. LS mean improvement at TAT was 10.83 points for ENJAYMO and 1.91 points for placebo.
Patients experienced significant and sustained improvements in anemia and fatigue at TAT
ENJAYMO had sustained treatment effect in CAD with long-term use over 1.5 years1,2
FACIT-Fatigue scores over time in CADENZA (mean)
Study Limitation: Patient-reported fatigue after TAT may be an under- or overestimation due to the open-label design and lack of a comparator arm during the extension period (6 months-1.5 years). Interpret these data with discretion given the small sample size and descriptive statistics.
Baseline: 31.67 | Month 6 (TAT): 43.15 | Year 1.5: 43.25
‡‡LS mean improvement in FACIT-Fatigue Scores vs placebo at TAT was significant (treatment effect: 8.93, 95% CI: 4.0-13.9, P<0.001), with scores nearing the general population (43.6 mean).1,4
A well-tolerated safety profile studied over 2.5 years1,2
ENJAYMO safety was evaluated in CADENZA, a 6-month placebo-controlled study (Part A [n=42]), followed by a 1 year open-label, single-arm study (Part B [n=39]) and CARDINAL (an open-label, single-arm study [n=24])
Adverse reactions (≥10%) in patients receiving ENJAYMO with a difference >5% vs placebo (CADENZA Part A)
| Adverse reactions | ENJAYMO (n=22) |
Placebo (n=20) |
|---|---|---|
|
Headache
Hypertension Rhinitis Acrocyanosis Raynaud's phenomenon |
5 (23%) 5 (23%) 4 (18%) 4 (18%) 4 (18%) |
2 (10%) 0 0 0 0 |
No meningococcal infections were reported with ENJAYMO
- ENJAYMO may increase susceptibility to serious infections, including infections caused by encapsulated bacteria such as Neisseria meningitidis (any serogroup), Streptococcus pneumoniae, and Haemophilus influenzae
Serious adverse reactions were reported in 2 patients
- Serious adverse reactions were Raynaud's phenomenon (n=1) and febrile infection (n=1)
Only 2 patients discontinued due to adverse reaction
- Adverse reactions leading to discontinuation were Raynaud's phenomenon (n=1), acrocyanosis (n=1), and infusion-related reactions (n=1)
Most common adverse reactions
- The most common adverse reactions (≥18%) reported were rhinitis, headache, hypertension, acrocyanosis, and Raynaud’s phenomenon